EXPANDING THE NEWBORN SCREENING FOR BILIARY ATRESIA USING DIRECT BILIRUBIN: AN IMPLEMENTATION SCIENCE STUDY
Tebyan Rabbani1, Jay Shah2.
1Gastroenterology , Stanford , Palo Alto , CA, United States; 2Gastroenterology , UT Health San Antonio, San Antonio, TX, United States
Introduction
Infants with biliary atresia (BA) have the best outcomes when identified early and the Kasai portoenterostomy (KPE) is performed before 45 days of life (DOL). However, the average age at the time of KPE in the United States is 63 days old. In our hospital system, the average age of KPE was performed 60 DOL. To address the problem of late presentation, we implemented a BA screening strategy utilizing fractionated bilirubin.
Methods
Multiple education campaigns were made to inform interdepartmental staff and outside PCPs of this new study. New institutional policies were established that all newborns were tested at 24 hours of life (Stage 1), and those with levels ≥0.5 mg/dL were followed further. The infant’s PCP was contacted to recommend a repeat fractionated bilirubin at 2 weeks of life (Stage 2). If the repeat DB was >1.0 mg/dL, the patient was evaluated by GI.
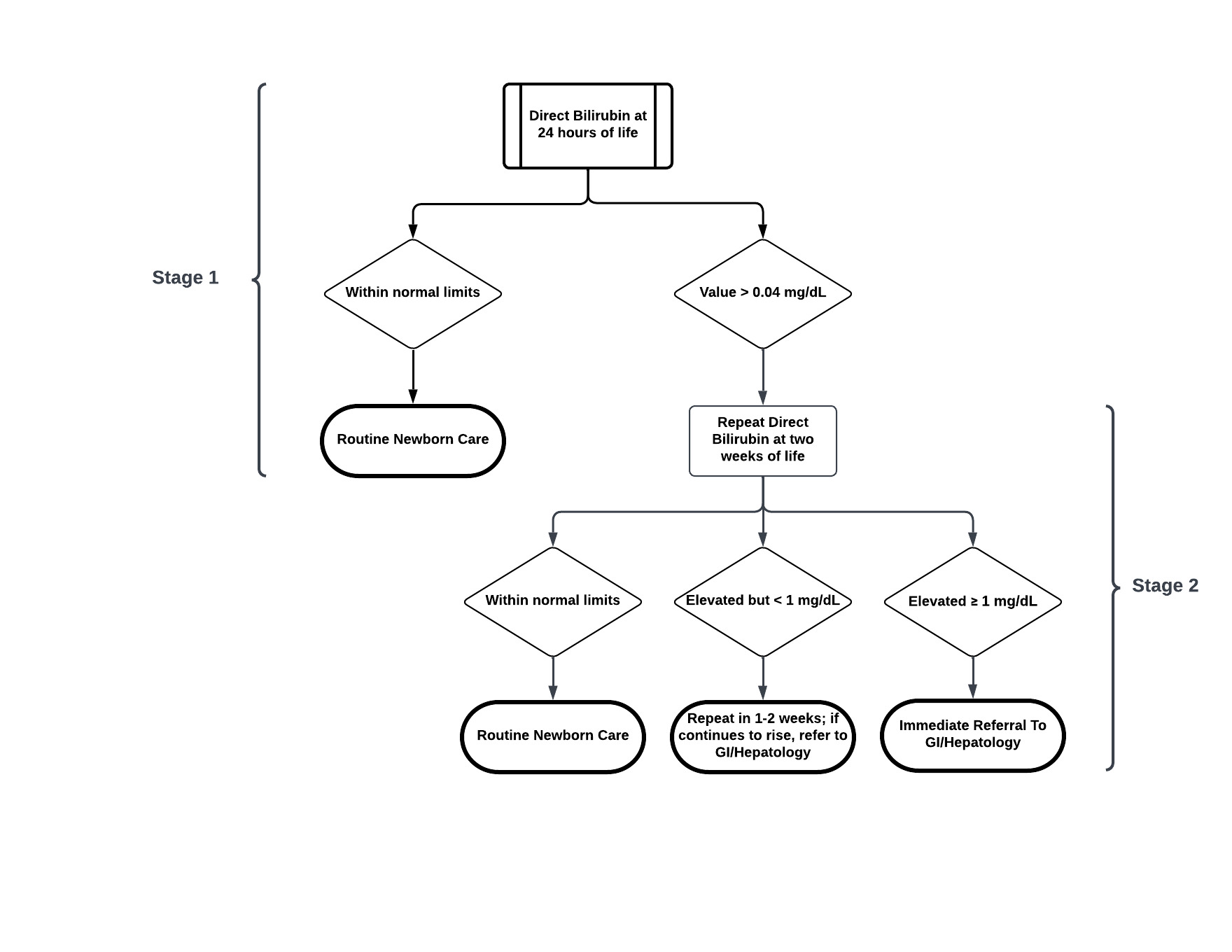
Results
3861 (99.5%) were screened. There were 40 infants (1%) who had DB levels ≥0.5 mg/dL initially. Upon repeat testing at 2 weeks, there were 3 groups of infants: not retested (n=1), retested ≤1 mg/dL (n=40), and retested >1 mg/dL (n=12) (Table). The average age at retest was 14 DOL and for those with levels >1 mg/dL, the average time to be seen by GI was 4.3 days. The range of DB for infants that were negative at Stage 2 was 0.2 – 0.8mg/dL. The range of DB for infants that were positive at Stage 2 was 1 – 9.6mg/dL. 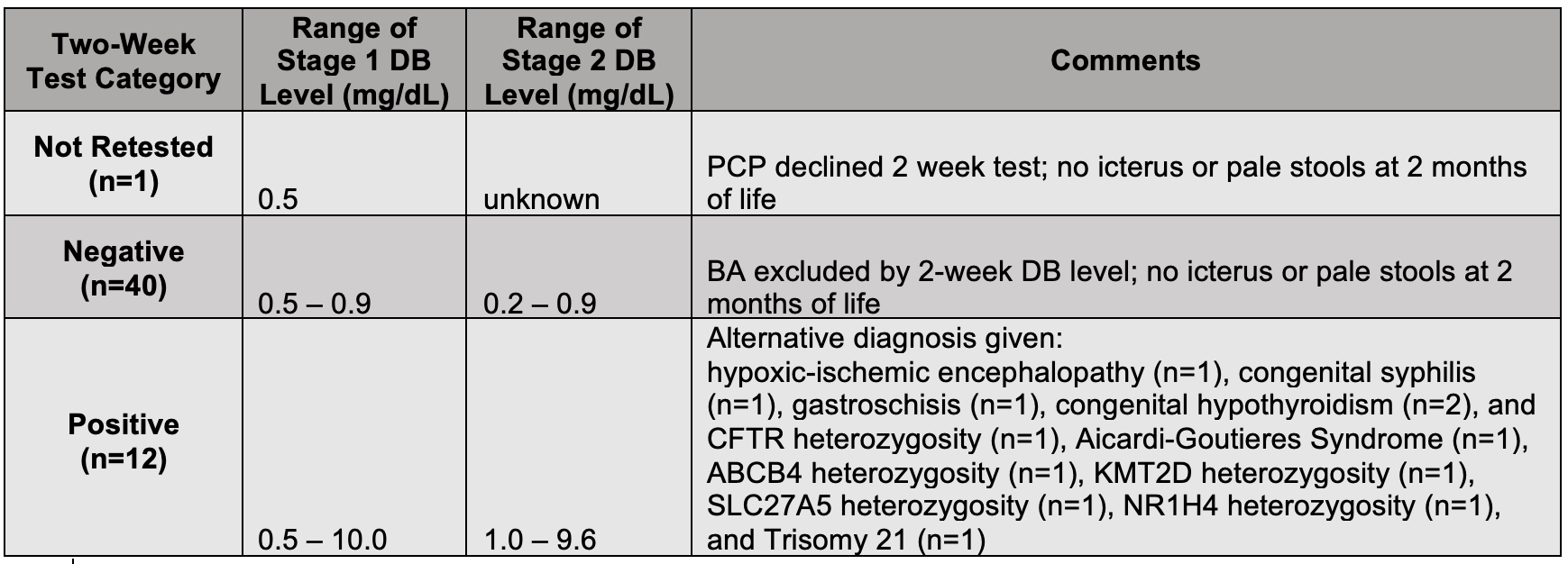
Conclusion
Implementing the screening at our hospital included: (1) Education campaigns to inform interdepartmental staff and local PCPs, (2) Changing order sets and note templates, (3) Establishing direct bilirubin reference intervals, (4) Designing educational pamphlets for families, (5) Designing a method to review Stage 1 results, (6) Informing PCPs of positive Stage 1 results, (7) Following up Stage 2 results, (8) Referral to GI. Screening had a net false positive rate of 0.3% (12/3861) and identified causes of cholestasis other than BA as well. BA was excluded by 28 DOL. Our results can provide a template for institutions interested in implementing a BA screening protocol in their practice.
If you have any questions during the meeting, please go to the registration desk. Our emails will be monitored sporadically.
REGISTRATION DESK OPENING TIMES
Sunday, October 15, 16:00-18:00 Monday, October 16, 07:00-18:00 Tuesday October 17, 07:00-12:30