Lack of differences in safety and effectivity among three induction immunosuppression protocols during the first-year post-liver transplantation in pediatric patients. A multicenter study.
Alejandro C Costaguta1, Guillermo Costaguta2, Daniel D'Agostino3, Gabriel E Gondolesi4, María Belén Pallitto3, Carolina Rumbo4, Oscar Bottasso5, Fernando Álvarez2,6.
1Liver Transplantation Unit, Sanatorio de Niños, Rosario, Argentina; 2Gastroenterology, Hepatology and Nutrition, CHU Sainte-Justine, Montreal, QC, Canada; 3Gastroenterology, Hepatology and Nutrition, Hospital Italiano de Buenos Aires, CABA, Argentina; 4Multiorgan Transplantation Unit, University Hospital Fundación Favaloro, CABA, Argentina; 5IDICER, CONICET, Rosario, Argentina; 6Department of Pediatrics, Montreal's University, Montreal, QC, Canada
Introduction: Immunosuppression varies among centers. Few comparative studies are published defining the best evidence-based approach. Pediatric patients are ideal to explore differences being a more homogeneous population with a lower rate of comorbidities
Methods: A retrospective study of patients receiving first liver transplantation in the four participating centers between January 2015 and 2019 was conducted. Patients were classified based on the immediate post-transplant immunosuppression in Group A (Basiliximab + Steroids + Tacrolimus), B (same as A + Thymoglobulin), and C (Steroids + Tacrolimus). Patients with other schemes were excluded. Main variables were incidence of rejection, infections, and first-year patient and graft survivals. A sub-set of Biliary Atresia patients was carried out to assess a more homogeneous population. GraphPad was used for statistical analysis; a p-value of 0.05 was considered significant.
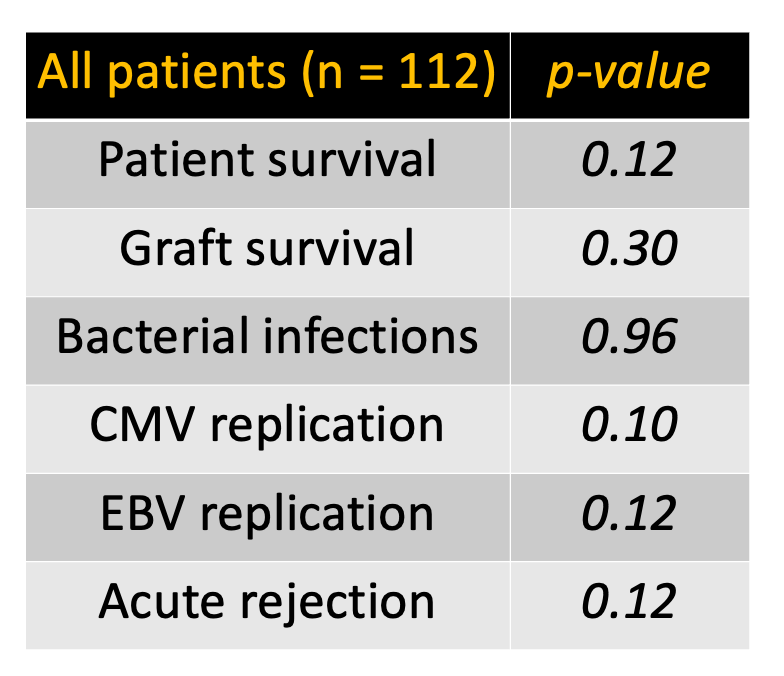
Results: 97 patients from 4 centers were recruited (Group A n= 52, Group B n= 25, Group C n= 20). Proportion of living donors were similar (p=0.93). No differences in rejection (p=0.12), CMV (p=0.10) or EBV (p=0.12) replication, nor other viral or bacterial infections (p=0.96) were noted. Patient (p=0.12) and graft (p=0.30) survival were similar (Table ). Results were similar among biliary atresia except for CMV replication, more frequent in group B when compared to Group C (p=0.04). Patient and graft survival were 100% in all groups.
Conclusion: Results are comparable regardless of the immunosuppression protocol in this series. Since our study has been retrospective, a multicenter prospective properly powered study would be required to validate this conclusion.
If you have any questions during the meeting, please go to the registration desk. Our emails will be monitored sporadically.
REGISTRATION DESK OPENING TIMES
Sunday, October 15, 16:00-18:00 Monday, October 16, 07:00-18:00 Tuesday October 17, 07:00-12:30